World Hepatitis Day 2025: Mapping the Hepatitis Development Pipeline
While liver diseases encompass a wide range of conditions, including steatohepatitis and autoimmune hepatitis, World Hepatitis Day places a dedicated spotlight on viral hepatitis, a group of infections that remains one of the leading causes of liver-related illness and death worldwide.
Despite decades of progress, viral hepatitis continues to pose a major global health challenge, underscoring why its elimination is a cornerstone of the World Health Organization’s 2030 public health agenda.
Although World Hepatitis Day is on July 28, we’re highlighting it a few days early to help raise awareness and spark timely conversations.
Key facts about viral hepatitis
Viral hepatitis is caused by five primary viruses: A, B, C, D, and E.
- Hepatitis A (HAV) and E (HEV) generally lead to acute infections, are vaccine-preventable, and often self-resolve, though treatment options are limited and HEV vaccination is only available in select regions.
- By contrast, Hepatitis B (HBV) represents the most common form of chronic viral hepatitis, and remains a significant global burden. Although vaccination can prevent HBV infection, a definitive cure has yet to be achieved, even with currently available antivirals.
- Hepatitis D (HDV), which only occurs in those co-infected with HBV, is considered the most severe form due to its accelerated disease progression. It can be prevented through vaccination against HBV, but therapeutic options remain limited.
- Meanwhile, Hepatitis C (HCV) stands apart: it has no vaccine, but is now considered highly curable thanks to the success of modern antiviral therapies.
These distinctions in pathogenesis, prevention, and treatment shape the diverse and evolving strategies within the hepatitis drug development landscape.
What Does The Viral Hepatitis Drug Pipeline Look Like?
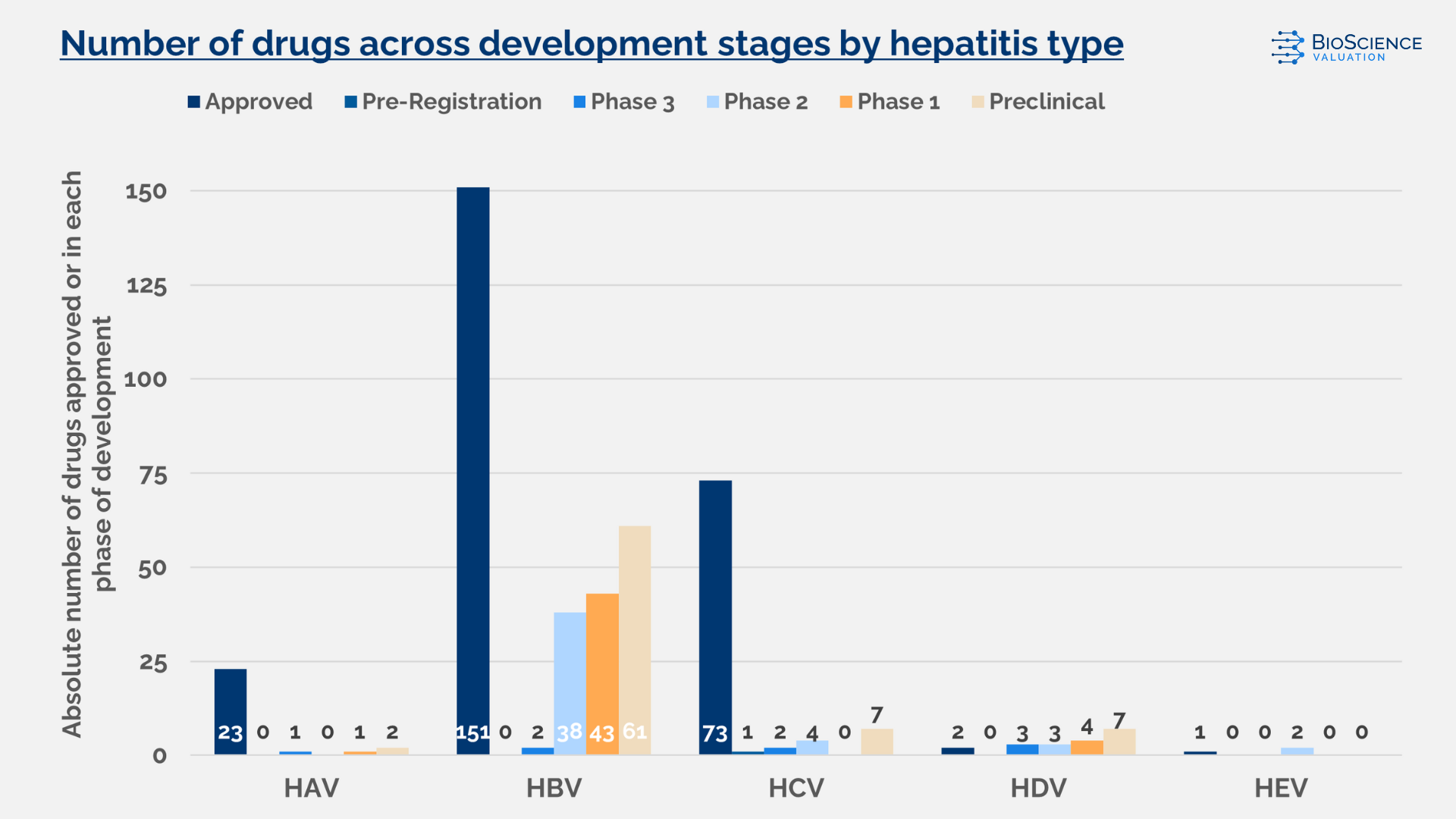
HBV currently has the most active development pipeline, with nearly 150 candidates in preclinical and clinical stages. Figure 1 illustrates the total number of drugs in development and those already approved.
As shown in Figure 2, HBV and HDV have the most diverse pipelines, with candidates spread across all phases of clinical development. HCV also shows a diverse pipeline, but with fewer development candidates in total compared to HBV (in fact, the number of assets in development is similar to HDV).
Types Of Intervention Across Hepatitis Types
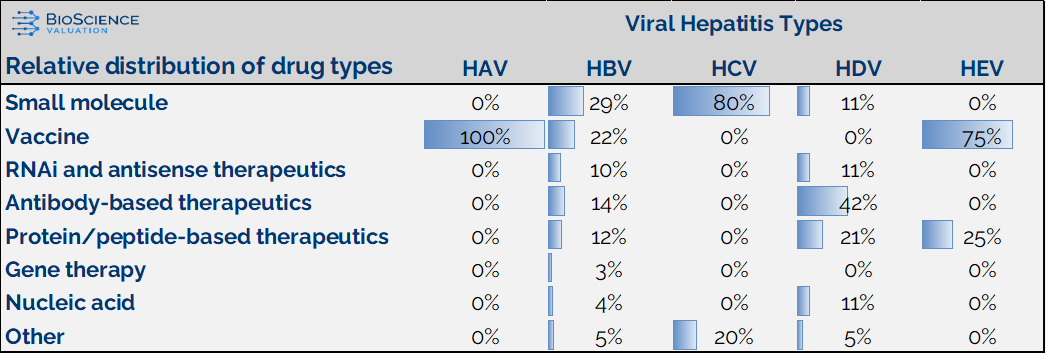
To better understand the evolving landscape, we categorized drug development efforts, both prophylactic and therapeutic, into seven major drug classes (see table below). These categories reflect the unique biology of each virus as well as strategic priorities in development.
- For HAV and HEV, the pipeline is vaccine-focused, aligning with their typical acute and self-limiting nature.
- In contrast, HBV shows the broadest range of drug classes, from small molecules and therapeutic vaccines to RNA-based treatments, monoclonal antibodies, and even gene therapies. This highlights a drive for a functional cure beyond simple viral suppression.
- HCV’s development remains dominated by small molecule antivirals (about 80%), reflecting its success story in antiviral innovation.
- Meanwhile, HDV is largely focused on biologic therapies.
This variation in drug development strategies reflects not only the scientific complexity of each virus but also highlights where the greatest unmet needs and future opportunities lie.
As the global hepatitis landscape evolves with advancing therapies and prevention strategies, the critical question remains: how can we accelerate innovation and equitable access to ultimately achieve the WHO’s ambitious goal of eliminating viral hepatitis as a public health threat by 2030?
For biotech and pharma teams working toward that vision, or developing breakthroughs in other fields, we offer the clarity and support needed to move forward with confidence. Please don't hesitate to reach out!
Links and sources: